Viscosity and surface tension are both important fluid properties. Both play a key role in product development (ex: detergents, inks, biopharmaceuticals, injectables, food and beverages), however both affect fluids and development differently.
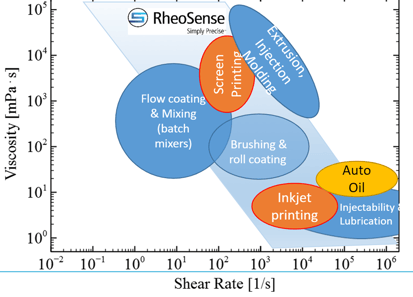
Viscosity is a fluids resistance to flow, often referred to as the "thickness" of a fluid. At a molecular level, viscosity is a result the interaction, or friction, between the different molecules in a fluid. While that seems straight forward, viscosity is complex and often confusing concept. First, there are many types of viscosity - dynamic, kinematic, steady sheer, relative, extensional - and understanding what they are is only the first piece of the puzzle. Type of fluid, temperature, flow conditions, and pressure are all factors affecting viscosity. When considering all the complex factors that influence viscosity, this seemingly simple concept becomes complex quickly.
When measuring viscosity, it is important to know if the fluid you are working with is Newtonian or non-Newtonian. Viscosity of Newtonian fluids is constant and therefore independent of shear rate whereas the viscosity of non-Newtonian fluids is not constant and therefore dependent on shear rate. In real world applications, most fluids are non-Newtonian, which means that their viscosity is dependent on shear rate (Shear Thinning or Thickening) or the deformation history (Thixotropic fluids).
Compared to viscosity, surface tension is a much simpler concept. Surface tension is the tendency of liquid surfaces to shrink to the minimum surface area possible. This tends to be a more static phenomenon than viscosity and is changed mostly by either temperature (as temperature decreases, surface tension increases), or addition of substances that modify molecular bonding characteristics (such as surfactants). Surface tension is an important factor for R&D teams when formulating and testing quality of products involving fluids.

We often relate liquids with high viscosity as having high surface tension as well, however this correlation is not accurate. Examples of high viscosity, low surface tension fluids include substances such a s glue. Because surface tension is caused by surface molecule forces and viscosity is related to internal friction of molecules it can be noted that there is no direct correlation between the two fluid properties. While no direct correlation exists between viscosity and surface tension, there is a existing relationship between the two - surface tension will vary as a direct result of viscosity at a constant temperature. Both properties affect product quality (such as inkjet printing, paint rolling, detergent formulation, and drug development), and are properties of a surfactant solution that exhibit an abrupt change at the critical micelle concentration (CMC).
Written by: Eden Reid, RheoSense Senior Marketing and Sales Operations