The antibody therapeutics field is expanding and significant research efforts are being heavily invested. One of the most labor intensive and costly challenges in development includes the difficulty in formulating therapeutically exceptional antibodies due to high viscosities.
High viscosities of the candidate drugs can lead into disqualification or costly development of alternative delivery method, due to the difficulty of injection for high viscosity medications without effecting efficacy or causing patient pain during administration. Because of these challenges caused by higher viscosities, drug stability in mAb formulations must be achieved with the lowest viscosity values possible. For example, it has been known that variation in the important yet small CDR regions of antibodies can significantly affect the viscosity of formulation. Referring to Figure 1, below, the viscosity behavior of monoclonal antibodies (mAbs) can differ dramatically based on concentration.
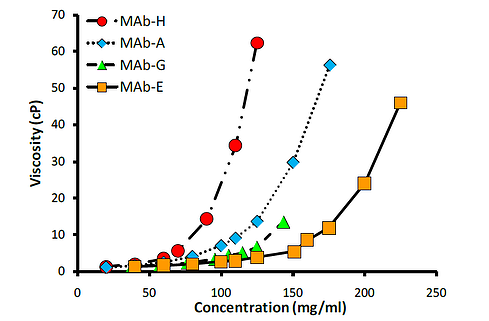

Figure 1. Viscosities of mAb-h rapidly increases with rising concentration, and mAb-h would thus be the least favorable candidate as an injectable. (Modified from Yadav et al., 2010, J Pharm Sci 99:4812-29.)
Proper design of antibodies and bioprocess to produce candidate proteins at an early stage with precise viscosity control would benefit. Therefore, it is as important to measure the viscosities of the candidate drugs and drug injectability at a very early stage of development for qualification in order to maximize the return on investment. In particular, small sample volume viscosity measurement is essential at the early stage as candidate drugs are available only in small quantity.
Published April, 2015 in GEN BioProcessing, VROC® technology — microfluidic rectangular slit method, is featured in the tutorial focusing on measuring small sample viscosities of antibody formulation with m-VROC® viscometer and how viscosity measurements at high shear rates are correlated to Injectability.

Proper design of antibodies and bioprocess to produce candidate proteins at an early stage with precise viscosity control would benefit. Therefore, it is as important to measure the viscosities of the candidate drugs and drug injectability at a very early stage of development for qualification in order to maximize the return on investment. In particular, small sample volume viscosity measurement is essential at the early stage as candidate drugs are available only in small quantity.
Published April, 2015 in GEN BioProcessing, VROC® technology — microfluidic rectangular slit method, is featured in the tutorial focusing on measuring small sample viscosities of antibody formulation with m-VROC® viscometer and how viscosity measurements at high shear rates are correlated to Injectability.

COMMENTS

